Frequently Asked Questions
Diagnostic Products
What is the stability and storage requirements for products?
All of our ZeptoMetrix NATtrol™ products are refrigerator stable (2-8C). Other products may have different requirements and should be stored as indicated on the Package Insert (PI). If stored properly products are stable through the expiration dates printed on each vial / kit.
Is the NATtrol™ Control/Panel infectious?
NATtrol™ products are non-infectious. Good laboratory practices recommend using Universal Precautions when handling these products as suggested.
How do I use the Control?
NATtrol™ controls should be used per the information found in product documentation and/or as guided by diagnostic platform suppliers.
What is the stability and storage for titered bacterial culture material?
Expiration date is 2 years from date of manufacture and must be stored frozen at -65C or below. These products are shipped frozen on dry ice. One freeze thaw cycle is acceptable.
Can I quantitate controls?
Many of the NATtrol™ products are qualitative and are listed as such in our product documentation. External Run Controls and Linearity Panels are quantitative and this information can be found in the appropriate product documentation.
What do I need to order live culture material (microorganisms)?
Live cultured material is infectious and requires a signed Material Transfer Agreement (MTA).
What is the shelf life of NATtrol products?
NATtrol products have a shelf life ranging from 3 to 24 months from the date of manufacture
Analytical Reference Materials
What technique do you use to certify your PO4 for IC Standard? Do you use an acid-base titration? If so, how do you make sure the standard is certified for PO4 only?
Our stock Ion Chromatography Certified Reference Materials are certified by averaging the values obtained by classical wet assay and ion chromatography analysis. The wet analysis method for PO4 stock solutions is precipitation using magnesia mixture. Filter, ignite, and weigh as Mg2P2O7. The ion chromatography result is traceable to NIST SRM 3186.
I am using SPEX certiprep standards to analyze pesticide products on a UPLC using a PDA detector. For all of the different standards, there is a large peak that comes off prior to 1 minute into the injection. What is that? It doesn't show up in any of our samples.
First guess is that your standard matrix and your mobile phase matrix are not matched or not perfectly matched and you are seeing a solvent matrix peak or matrix differential peak. These types of peaks are most often found in the dwell volume of your HPLC column and show up as unretained peaks usually within the first 30 seconds to 2 minutes of a run depending on column size, tubing dimensions and lengths. Do you know what your dwell time is for your system? If it is around that one minute mark then it is most likely that solvent peak. It often can be eliminated or reduced by matching the solvent in the samples and standards to the same solvent conditions of the mobile phase. Another possibility is if you are looking at a mix of pesticides there could be some unretained pesticides that come out in that dwell time as well. Again, we can investigate your questions further if you provide us with the part numbers, lot numbers and additional run information.
What technique do you use to certify your PO4 for IC standard? Do you use an acid-base titration? If so, how do you make sure the standard is certified for PO4 only?
Our stock Ion Chromatography Certified Reference Materials are certified by averaging the values obtained by classical wet assay and ion chromatography analysis. The wet analysis method for PO4 stock solutions is precipitation using magnesia mixture. Filter, ignite, and weigh as Mg2P2O7. The ion chromatography result is traceable to NIST SRM 3186.
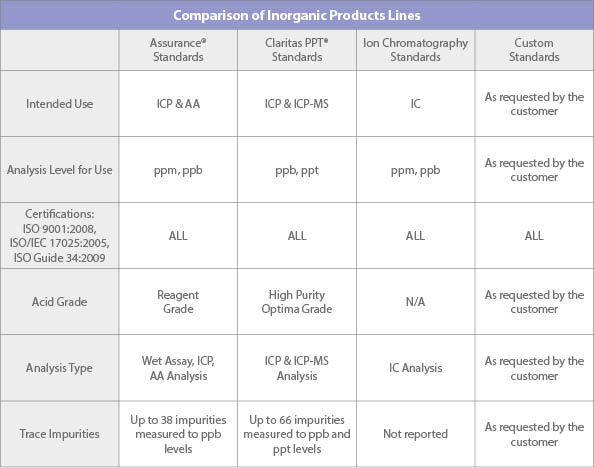
What are the differences between your various Inorganic Product Line offerings?
I am using SPEX CertiPrep standards to analyze pesticide products on a UPLC using a PDA detector. For all of the different standards, there is a large peak that comes off prior to 1 minute into the injection. What is that? It doesn’t show up in any of our samples.
First guess is that your standard matrix and your mobile phase matrix are not matched or not perfectly matched and you are seeing a solvent matrix peak or matrix differential peak. These types of peaks are most often found in the dwell volume of your HPLC column and show up as unretained peaks usually within the first 30 seconds to 2 minutes of a run depending on column size, tubing dimensions and lengths. Do you know what your dwell time is for your system? If it is around that one minute mark then it is most likely that solvent peak. It often can be eliminated or reduced by matching the solvent in the samples and standards to the same solvent conditions of the mobile phase. Another possibility is if you are looking at a mix of pesticides there could be some unretained pesticides that come out in that dwell time as well. Again, we can investigate your questions further if you provide us with the part numbers, lot numbers and additional run information.
We have a Perkin-Elmer ICP-OES, with a Meinhard nebulizer and a simple cyclonic chamber. I have two questions: 1. What is the best way to eliminate memory effects from mercury? Gold or mannitol (perhaps cysteine)? 2. Is ICP-OES recognized by the EPA for mercury reporting yet?
1) Our lab recommends using Gold (Au3+) for eliminating Hg memory effects as we have had success by doing so. In regards to question
2) This really depends on your detection limit requirement. We believe most EPA labs or CLP labs still largely use cold vapor (CVAFS) to analyze Hg.
Regarding your product arsenic AA standard (CAT# PLAS2-3X). Is the arsenic in the oxidation of 3+ or 5+. If it is in the 3+ form, would that affect my results if the active being analyzed is 5+ (arsenic pentoxide)?
This standard is prepared from Arsenic metal. Technically, as in this standard (PLAS2-3, 5% HNO3) is in its oxidation state of +5. If it is in the 3+ form, would that affect my results if the active being analyzed is 5+ (arsenic pentoxide)? No, As should be in +5 oxidation state in this standard although this standard is not guaranteed for existing As species. If you used an As3+ standard as a reference to analyze a sample containing As5+, will your analytical results be affected? This depends on the methods you used for determination as well as your testing specification (requirement) to meet. If you want a standard containing only As5+, you may want to try our catalog product SPEC-AS5.
The Effect of Matrices in the Analysis of Mercury by ICP.
The Effect of Matrices in the Analysis of Mercury by ICP. Bill Driscoll.
Mercury analysis is one of the most difficult analyses by ICP and ICP/MS instrumentation. Mercury standards are sold in nitric acid. However, nitric acid alone is not an ideal matrix for analysis. Diluted mercury standards in nitric acid matrix show a lot of variation when analyzed over a period of time. SPEX CertiPrep conducted a study on the effect of matrices in the analysis of Hg by ICP.
Solution preparation: NIST SRM 3131 was diluted to 100 mg/L in two different nitric acid concentrations, 10% HNO3 and 5% HNO3. These two solutions were further diluted to 2 mg/L on five different days in four different matrices as shown in Table 1.
Solution analysis: Initial calibration was done using 2 mg/L Hg in 20% HCl/1% HNO3. Final analysis was done on all the diluted samples at the same date following completion of analysis of a given matrix diluted on different days before taking up the second matrix.
Conclusion: Results are shown in Table 2: Concentration shown is the average of four replicates in three wavelengths, 253, 194 and 184 nanometers. Consistent analytical results are obtained when mercury is analyzed in 2% HCl + 1% HNO3 or in 20% HCl + 1% HNO3 when the different dilutions are analyzed over a period of time. Addition of gold to nitric acid matrix also produced repeatable results. F test showed no significant difference in the standard deviations between these matrices while the standard deviation was very significantly higher for the analysis in 2% nitric acid alone. It is concluded that nitric acid alone is not an ideal matrix for the analysis of Mercury.
Besides concentrations of elements, what is the difference between a calibration standard and QC check standard?
The process below outlines the difference between a calibration standard and a check standard:
1. Make an educated approximation as to the elements and their concentrations in your unknown sample.
2. You would buy a Certified Reference Material that contains the elements in the sample you'd like to analyze. This is your calibration standard.
3. Dilute the calibration standard to three or four different dilutions and run a curve.
4. Run another known standard (QC check standard) with concentrations within this range and calculate the recovery. Usually the lab defines what a suitable recovery should be depending on the regulations they adhere to.
5. Run your unknown sample.
6. Spike the QC standard at a similar concentration as your step 4. Verify the spike recovery.
What are the common contaminants being monitored in the manufacture of biodiesel?
Biodiesel consists of esters made from fatty acids that come from a reaction between vegetable oil and an alcohol in the presence of base and a little heat (transesterification). The possible contaminants are:
1. Unreacted vegetable oil consisting of mono, di, and triglycerides.
2. Methanol or another alcohol, left over from the reaction creating the methyl esters (actual biodiesel) from the vegetable oil.
3. Base, usually sodium or potassium hydroxide, which is the catalyst used to separate the glycerol molecule from the vegetable oil glyceride molecules.
4. Glycerol which usually settles to the bottom of the reaction vessel when biodiesel is made.
Can you give me instructions on how to improve my measurements for Sodium?
Instrument materials (test tubes, autosampler tubes, etc.) and clean lab techniques all can impact your Sodium measurements. Follow these guidelines for minimizing contamination and improving your Sodium measurements:
For test tubes, autosampler tubes, and other vessels:
- The best material is PTFE, followed by Polypropylene.
- Cleaning: Soak in 2-5% nitric acid for a few hours.
- Storing: Fill up with DI water, or 2-5% nitric acid.
ICP Tubing:
- Using the unused channel of the peristaltic pump, recirculate the blank solution through the pump tubes that are to be cleaned.
Nebulizer:
- Glass nebulizers contain Sodium. Use plastic or PTFE nebulizers instead.
- An ultrasonic nebulizer is more effective than a pneumatic nebulizer.
- PTFE direct injection nebulizers are also very useful.
Torch:
- Wash torch with DI water or 2% nitric acid (though dilute HF is ideal for cleaning, use of it is prohibited due to legal/regulatory/safety requirements).
- For sample introduction tubes (central tube of the torch), use alumina tubes.
Keep in mind, clean room environments and high grade chemicals are also extremely useful in reducing contamination.
Can you explain why the response for Acrolein in our calibration standard has gone down within two weeks?
Acrolein can dimerize, or break down, to form 3-methoxypropanol and dimethyl acetal depending on factors such as storage condition, other compounds is in the solution, the solution matrix itself and, of course, time. On a typical GC capillary column, the breakdown peaks will show up shortly after the Acrolein. If you have access to a GC/MS you can easily identify Acrolein, major ion 56 and distinct minor ion of 55. The breakdown product 3-methoxypropanol has the major ion of 60 and 45. The breakdown product dimethyl acetal has the major ion of 71 with minor ions of 75 and 41. Acrolein is more stable when used in a methanol matrix. We recommend fresh working calibration standards weekly.
Why is my lab having difficulties finding and analyzing the metabolite Heptachlor Epoxide?
Heptachlor was a common agricultural pesticide until the mid-1980’s when it was banned by the US in most agricultural applications. The problem is that Heptachlor is particularly persistent in the environment. The original form of Heptachlor is readily metabolized in the environment by plants and animals to forms of heptachlor epoxide. Heptachlor epoxide has two isomers: A & B. The A form breaks down quickly while the B form is more persistent. The B form of the isomer is often the isomer of concern when analyzing samples in the laboratory. The A form of the isomer has been readily produced for testing but the B form is not widely manufactured. Many laboratories have been using the A form in their analysis when in fact the B form of the epoxide is actually their target analyte. The two isomers can have different retention times when run on GC. If the system is calibrated for the wrong standard, the compound of interest could be misidentified or missed entirely. Since the two isomers have different fragmentation patterns by GC/MS it is possible to distinguish between the isomers in a sample by spectra rather than by retention time alone.
We checked our instrument’s calibration with an ICV solution. Twenty two of twenty five elements were above or below the expected concentration, only three of them were OK. What is the reason?
You are performing measurements below detection limit. You have a low sensitivity and your standard is not nebulized correctly. The reasons could be autosampler error, solution mishandling, broken pump tube and/or capillary, or zero or very low nebulizer flow. Check the instrument for these defects and try the measurement again.
We have a calibration standard containing Metribuzin and are seeing two peaks for this compound, can you explain this?
Metribuzin breaks down depending on factors such as storage condition, other compounds in the solution, the solution matrix itself and, of course, time. On a typical GC capillary column, CV-5 for example, the breakdown peak will show up shortly after the Metribuzin. If you have access to a GC/MS you can easily identify Metribuzin, major ion 198 and distinct minor ion of 214. The breakdown product has the same major ion of 198, but contains distinct minor ions of 211, 239 and 254.
What happens to bis-chloromethyl ether in water?
Bis-chloromethyl ether rapidly hydrolyzes and decomposes into HCl and formaldehyde. Air sampling of this compound has led to successful analysis methods. SPEX CertiPrep sells Bis-chloromethyl ether in iso-octane at 1000ug/mL (S-880).
What is the effect of acid on sample recovery?
Acid concentration influences the nebulization process. Concentration of acid in the sample and standard should match perfectly. Amount of acid in the aliquots used for dilution should be taken in to account and further amount should be added if needed to match the standard.
Why is it recommended to add acid when diluting reference standards?
The addition of acid is recommended to prevent the formation of hydroxides. Hydroxides have a tendency to absorb on the surface of storage containers, autosampler tubes and all parts of the sample introduction system. Hence nebulized aerosol droplets will not be a true representation of the standard itself and will lead to lower intensity readings which will cause erroneous results. This is true for the sample as well the standard and one needs to match the amount of acid in both.
How much acid is recommended?
The kinetics for formation of hydroxides depends on the element. Quantity of acid should be enough to keep the elements in solution. Any precipitation would disturb the composition of the solution and/or the nebulization.
As a general rule, standards in the ppb range need at minimum 1% acid. Low ppm range standards need 2% acid, and a level of 5% is recommended for concentrations greater than 100 ppm.
How do I know I added a sufficient amount of acid?
If you see some cloudiness or precipitation, it can be inferred that the amount of acid added is not sufficient.
Sometimes, the precipitation may not be visible but still can affect the measurements. Indication for this is an abnormality shown in intensity vs time. Usually, higher concentration of acid will resolve this problem. However, always match the concentration of acid in the sample and the standard.
Why should the acid concentration in the sample match the standard it is measured against?
The density, viscosity and surface tension of the matrix have considerable effect on the droplet size of the aerosol. Only fine droplets are transported to the plasma torch. Matching the acid concentration of the sample and the standard helps in transporting similar quantity of droplets (from both solutions) to the torch, thus improving the accuracy.
My wavelength shifts during measurement. What is the reason and how do I avoid it?
The temperature of the optic is not stable; the instrument needs a warm up time before performing the first measurement. For most instruments, the warm up time is usually 15-30 minutes. If it fluctuates even after sufficient warm up time, the temperature control in the instrument is not functioning properly. Call for service from the manufacturer.
I checked your ICV solution and processed the calculation with and without the use of internal standards. The concentration for Na, K, Li were not within the range if I used an internal standard. What is the reason?
The easily ionizable elements have very different reaction on the plasma electron concentration than the internal standards used. These lines are known as “soft lines”. For alkali metals such as those you have mentioned, you should select another internal standard, such as Cesium, or do not use an internal standard. When selecting the internal standard, consideration should be given to the ionization stage (ion or atom) and ionization energy and excitation energy of the analytes of interest. Also, the selected internal standard should not be part of the analytes of interest in the mix.
When measured on consecutive days, the intensity for P, Se, S and Zn tend to be much lower; while I don’t notice such change for other elements in the mix. What is the reason?
There are two possibilities for this:
- There is oxygen in the optical path. Oxygen absorbs the low wavelength photons more than the higher wavelengths, thus decreasing the intensity. Call the manufacturer for service.
- The optical system has a window, and it may be corroding. The longer wavelength photons can go through but the shorter ones are absorbed. Your option is to change the window(s).
How does the regular use of internal standards improve accuracy and reduce measurement variation?
If internal standard is selected correctly, it can compensate for fluctuation in the power outputted from the power source thus increasing the precision of measurements; it will also compensate for the variation in nebulization if it occurs.
What are the conditions that lead to a zero reading for a Certified Reference Material and how are they avoided?
The most common ways to resolve a zero-reading reference material are:
- Check the sample introduction system. The nebulizer should be clean and the tubing connecting the nebulizer, peristaltic pumps and auto-sampler should be tight.
- Check the spectrum of the element(s) in question. Verify the peak is centered.
- Check the blank for any contamination.
- If the CRM is diluted, use the appropriate matrix and adequate acids for dilution.
- Analyze the solution at different concentrations than the one used (preferably two times higher or lower).
What is Isopiestic distillation? Can it be used to purify acids?
Volatile acids such as Hydrochloric acid can be purified by Isothermal Distillation, also known as Isopiestic Distillation. Place an open beaker containing reagent grade acid and an open beaker containing high purity water within a closed system at room temperature. An empty desiccator can be used to create such a closed system. Acid vapors will be continuously transferred to the high purity water until an equilibrium is established between the two solutions. This should be accomplished in 12 to 24 hours creating high purity acid from the water, at a lower concentration than that of the original reagent grade acid, of course. This method can also be used to purify ammonia as well.
What is the best way to store ion chromatography standards?
As per our Certificate of Analysis, stock anion standards such as nitrate, nitrite, phosphate, sulfate, and others are guaranteed stable to ±0.5% of the certified concentration for a period of 1 year if materials are kept tightly capped and stored under normal conditions of temperature (50-80°F) and Humidity (30-80%). Refrigeration is not required. If for any reason the standards are refrigerated, bring the solution to room temperature before using.
What is the measurement error in ICP?
In general the instruments are accurate to within ±1%. Most of the time, you can further refine the measurements by replications and using an exact calibration standard matching the analyte concentration and matrix of interest.
How do I prevent my antimony oxide (Sb2O3) solution from becoming a gelatin when I dissolve it in tartaric acid?
This is a great question with a very easy solution. While Sb2O3 dissolves easily in tartaric acid and water, the solution is clear at first but a gelatin like substance can form over time. This is a form of mold, and adding a trace amount of nitric acid to the solution can prevent this.
Can you please provide some tips on calculating the concentration of an individual ion from an anion group?
Great question, here are a few conversion factors for our most popular IC Standards:
- 1000 µg/mL Nitrate = 226 µg/mL Nitrogen
- 1000 µg/mL Phosphate = 326 µg/mL Phosphorus
- 1000 µg/mL Nitrate-Nitrogen = 1000 µg/mL Nitrogen
- 1000 µg/mL Phosphate-Phosphorus = 1000 µg/mL Phosphorus
- 1000 µg/mL Nitrite = 305 µg/mL Nitrogen
- 1000 µg/mL Sulfate = 334 µg/mL Sulfur
- 1000 µg/mL Nitrite-Nitrogen = 1000 µg/mL Nitrogen
- 1000 µg/mL Sulfate-Sulfur = 1000 µg/mL Sulfur
To calculate the gravimetric factor of other ions or at other concentrations, the formula is as follows:
(Formula weight of the element / Formula weight of the ion) * Concentration of the Ion
What is green chemistry and are SPEX CertiPrep products “green”?
Researchers at the EPA have defined twelve principles of green chemistry. These principles are:
- Prevent waste Design safer chemicals and products
- Design less hazardous chemical syntheses
- Use renewable feedstocks
- Use catalysts, not stoichiometric reagents
- Avoid chemical derivatives
- Maximize atom economy
- Use safer solvents and reaction conditions Increase energy efficiency
- Design chemicals and products to degrade after use
- Analyze in real time to prevent pollution
- Minimize the potential for accidents
Paul Anastas and John Warner in Green Chemistry: Theory and Practice (Oxford University Press: New York, 1998).
SPEX CertiPrep is constantly revising its policies and procedures to include more green practices each year. We try to limit our production stocks to both ensure quality and reduce waste. Many of our organic products are created for our customers on-demand, which dramatically reduces exposure and waste. SPEX CertiPrep has designed our own acid purification still to purify our own acids.
Our company has active programs to promote reduction, reuse and recycling of resources and materials.
SPEX CertiPrep is committed to increasing green practices and our own environment impact in 2009. Check back with us throughout the year to see updates on our progress!
What is melamine and why is it in the news?
Melamine is a small man-made, nitrogen-rich molecule which has been in use since it was first synthesized in 1834. Melamine has been combined with formaldehyde to form melamine resin which is used in countertops, whiteboards, flame retardants and home goods.
The scare of the past two years has actually come from melamine and a group of other associated chemicals: ammeline, ammelide and cyanuric acid. The illegal uses of these compounds as counterfeit protein substitutes in food products have caused illness and death in people and animals.
Proteins are organic molecules which contain, among other elements, nitrogen. Testing of many food items for protein involves the calculation of the nitrogen content to determine the amount of protein. Manufacturers from China added melamine and cyanuric acid to boost the nitrogen level of their products and therefore artificially elevate the protein levels of their products.
Unfortunately, melamine and cyanuric acid readily form together a complex network of bonds called melamine cyanurate. This new, very large molecule becomes a physical obstruction in body tissues and organs such as the kidneys. The crystals build up in the systems and shut the organs down. Melamine cyuranate is actually more toxic than either melamine or cyanuric acid alone.
The new awareness of the potential contamination of food stocks has prompted more laboratories to test for melamine, cyanuric acid and their associated compounds. SPEX CertiPrep has several standards for these compounds:
- S-4806: Melamine (500 µg/mL)
- S-4807: Cyanuric Acid (1000 µg/mL)
- S-4808: Ammeline (1000 µg/mL)
- S-4809: Ammelide (100 µg/mL)
- XQ-4042: All four compounds (1000 µg/mL)
What are the most common phthalates used in plastic products?
Phthalates are esters of 1,2-benzenedicarbolxylic acid. These compounds are used in almost every industry as coatings, binders, lubricants, plasticizers, and many other applications. The most common phthalates can be found in the table below.
The most widely used phthalates in production today are DEHP, DBP, and BBP Di-(2-ethylhexyl) phthalate (DEHP) is found in the production of polyvinyl chloride (PVC). DEHP is also widely used in the medical device industry. Plastics can contain up to 40% DEHP. Butyl benzyl phthalate is found as a plasticizer in vinyl foams, floor tiles, synthetic leather, and food production line conveyor belts. Dibutyl phthalate (DBP) is found in adhesives, binders and inks.
Phthalates have come under scrutiny in the past decade for their widespread contamination in the environment and their potential health consequences. SPEX CertiPrep has several phthalate standards in stock for your laboratory’s standard needs. Check back with us soon for our latest studies on phthalates in water samples!
What are the different types of plastics in use and what do the recycling numbers mean on the plastic packages? Which containers may contain Bisphenol A?
There are seven common plastic packaging materials. These food packaging materials each have a different recycling number called a “Plastic Identification Code” (PID). The codes are as follows:
PIC Abbreviation Compound Uses
- PET Polyethylene Terephthalate Soft drink and water bottles
- HDPE High Density Polyethylene Milk bottles, retail bags
- PVC Polyvinyl chloride PVC pipe, plastic films & wraps
- LDPE Low Density Polyethylene Squeeze bottles, cling wraps, food bags
- PP Polypropylene Microwave containers
- PS Polystyrene Foam containers
- Other Often polycarbonate Refillable bottles, baby bottles, other
Bisphenol A is most commonly found in polycarbonate materials such as baby bottles, sports bottles and reusable drink containers marked with the identification code “seven”.
SPEX CertiPrep has a Bisphenol A (S-509) standard available for your testing needs. Check back with us soon to see our study on Bisphenol A found in beverage containers!
What is MFSTA?
MFSTA is a reagent used for derivatizing compounds in order to detect them on a GC. The chemical name is N-trimethylsilyl-N-methyl trifluoroacetamide. It falls in the category of Silyation reagents which changes the molecule making it more volatile so that in can be analyzed. There are many types of derivitizing reagents. Most reagents fall into the categories of Acylation, Alkylation, Esterification and Silylation.
MFSTA is commonly used for glyceride analysis in biodiesels as well as for melamine in food raw materials.
Does vinyl acetate have stability problems in calibration standards?
Yes, vinyl acetate breaks down into acetic acid methyl ester (CAS# 79-20-9) in the presence of methanol and other compounds like ketones. Acetic acid methyl ester is also known as methyl acetate and has the characteristic m/z ions or 43, 59 and 74. SPEX CertiPrep supplies vinyl acetate (S-3800) as a single component standard, but be careful what you blend it with as it will not last long.
How stable is Anthracene?
Not as stable as you might think. Phenanthrene and Anthracene are isomers; Phenanthrene is stable while Anthracene is not. We have observed hundreds of samples in our past experience where the concentration of Phenanthrene is much higher than that of Anthracene. It ends up that Anthracene breaks down to form 1,4-Anthraquione. We have observed this happening in sealed ampules of calibration standards containing Anthracene. The breakdown reaction takes place over a 4-5 year period when sealed in an amber glass ampoule.
For a more detailed explanation of why this is, you might find this paper of interest.
We have a calibration standard containing Metribuzin and are seeing two peaks for this compound, can you explain this?
Metribuzin breaks down depending on factors such as storage condition, other compounds in the solution, the solution matrix itself and, of course, time.
On a typical GC capillary column, 5% diphenyl, 95% methylpolysiloxane stationary phase for example, the breakdown peak will show up shortly after the Metribuzin. If you have access to a GC/MS you can easily identify Metribuzin, major ion 198 and distinct minor ion of 214. The breakdown product has the same major ion of 198, but contains distinct minor ions of 211, 239 and 254.
What lithium borate flux would you recommend for dissolving calcined alumina?
A balanced flux can be a good alternative if you are treating samples of varying composition. With high alumina content, you can go higher in lithium metaborate proportion. We would recommend using either 50/50 or even 35/65 LiT/LiM flux for your samples of alumina. Also, we tend to favorize non-wetting agent already pre-fused in the flux for maximum efficiency for LiBr or LiI (49.75/49.75/0.50 LiT/LiM/LiBr or LiI, idem for 34.83/64.67/0.50). Bromine can interfere with alumina at low concentration, which means you should be fine, but if you prefer to avoid it, use LiI.